250
acute ischemic stroke patients
40 SITES
from US and New Zealand
CAUTION: Investigational device. Limited by federal (or United States) law to investigational use.

Transforming Intervention
The SUMMIT MAX trial is currently underway to evaluate the safety and effectiveness of the Monopoint® Reperfusion System. The platform was designed to streamline delivery and enable super-bore aspiration at the most common stroke targets.
250
acute ischemic stroke patients
40 SITES
from US and New Zealand
Effectiveness
Proportion of subjects with successful revascularization defined as a modified Thrombolysis in Cerebrovascular Infarction (mTICI) score of 2b or greater without adjunctive therapy.
Safety
Incidence of all symptomatic intracranial hemorrhage (sICH) within 24 hours post procedure.
Unlike any other system, each component of the Monopoint® platform is designed to nest inside the next, providing a streamlined unit that can track through curvatures without catching side branches.
The telescoping design with tapered components:

Balancing stability and flexibility, the Monopoint® platform allows you to deliver large (.070) and super-bore (.088) catheters to the distal M1.

Provides access and a stable base for delivering large-bore and super-bore catheters from a single rotating hemostatic valve (RHV).
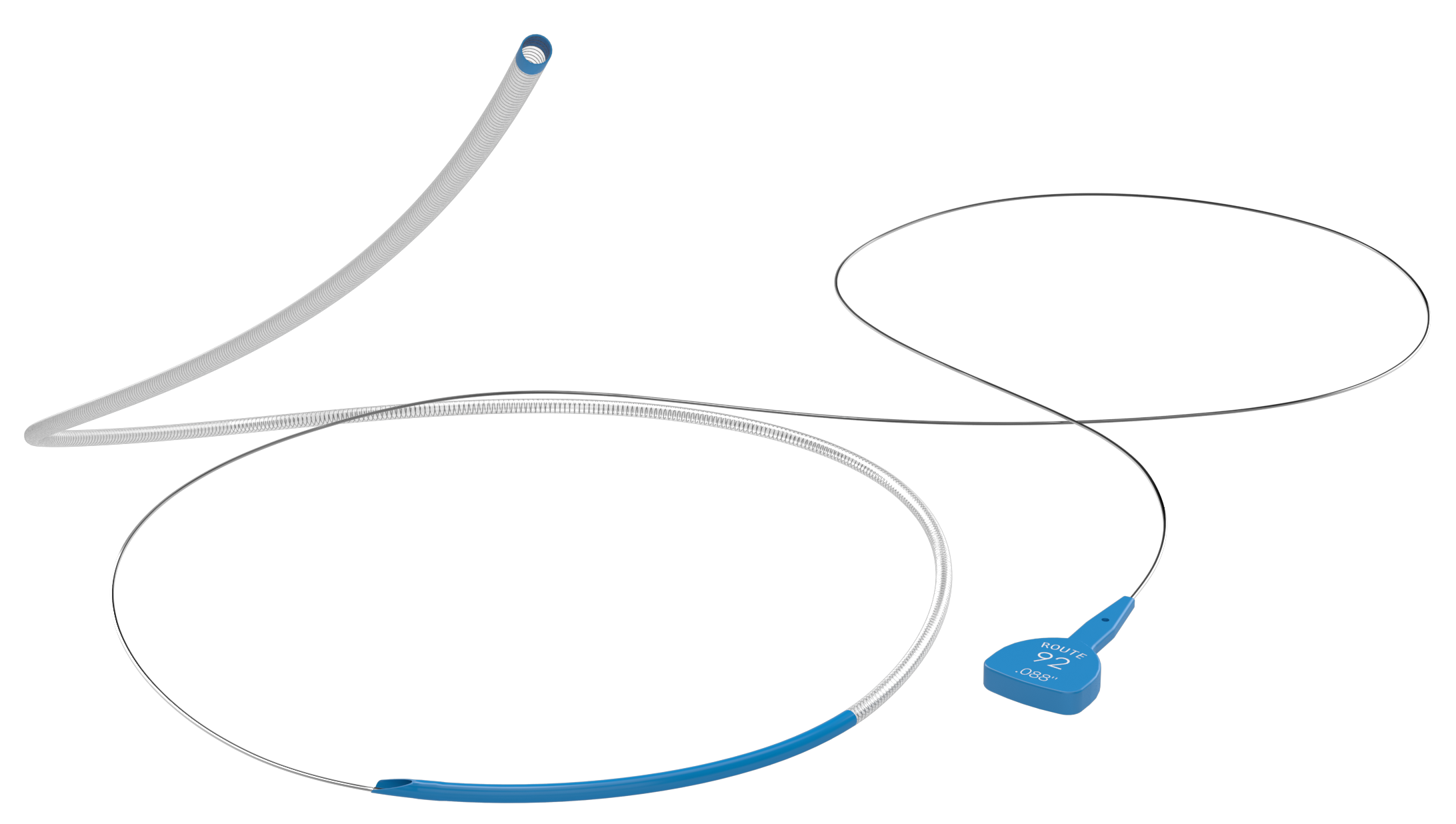
Hybrid design — engineered with a unique rail to reduce catheter-on-catheter friction and enhance operator control.
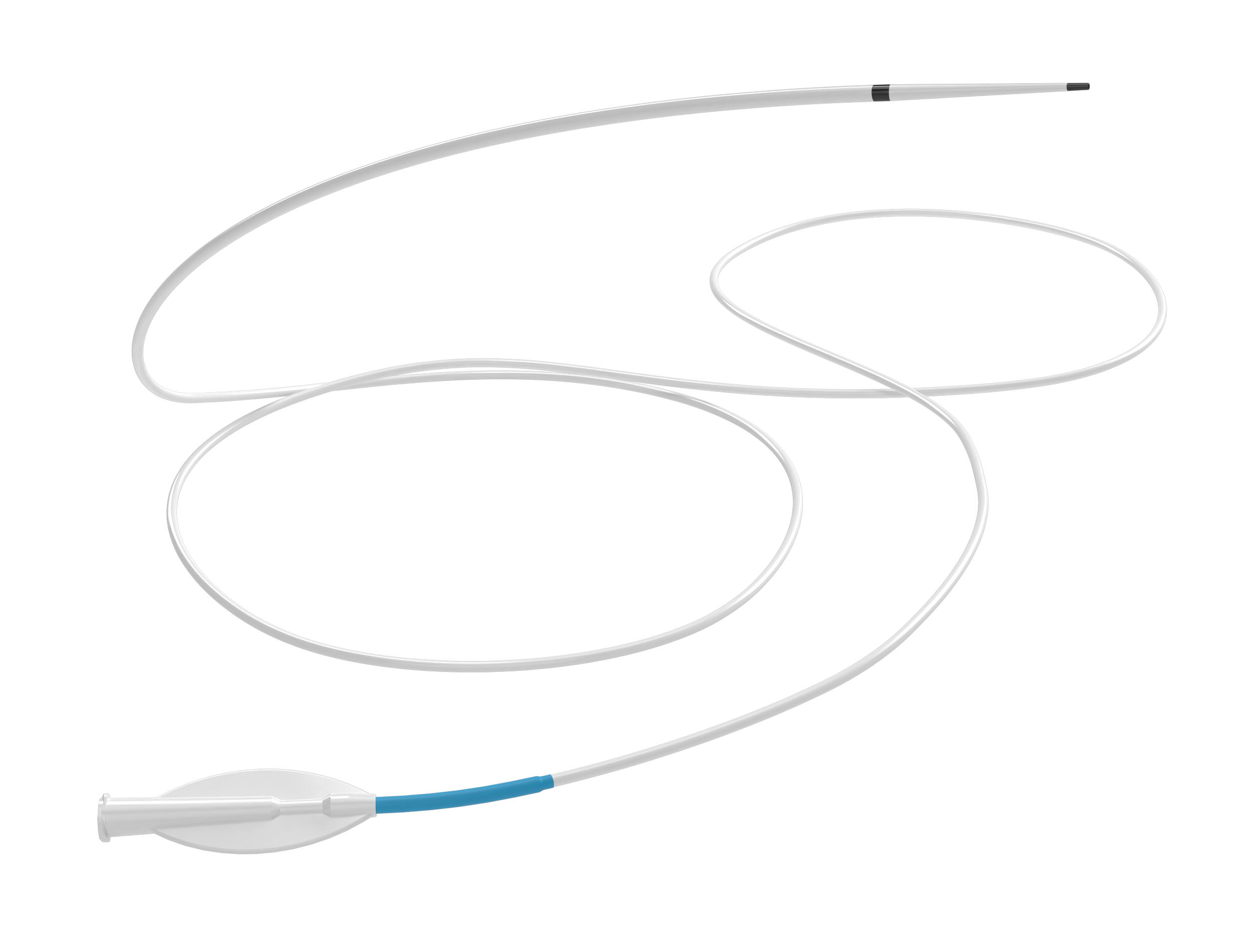
Tapered, atraumatic tip designed to follow the natural flow of blood and seek the primary vessel.
Want to learn more? Email [email protected]
CAUTION: Investigational device. Limited by federal (or United States) law to investigational use.